So that you might better understand the procedure for
calculating water potential, here is a practice problem.
Once you know the solute concentration, you can
calculate solute potential using the following formula:
Solute potential (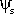 )
= –iCRT
)
= –iCRT
| i = |
The number of
particles the molecule will make in water; for
NaCl this would be 2; for sucrose or glucose,
this number is 1
|
| C = |
Molar concentration
(from your experimental data)
|
| R = |
Pressure constant =
0.0831 liter bar/mole K |
| T = |
Temperature in
degrees Kelvin = 273 + °C of solution.
Assume room temperature is = to 22 oC. |
The molar concentration of a sugar solution in an
open beaker has been determined to be 0.3M. Calculate
the solute potential at 27 degrees. Round your answer to
the nearest hundredth.
The pressure potential of a solution open to the air is
zero. Since you know the solute potential of the
solution, you can now calculate the water potential.
Notes to help answer questions.
1. Draw a straight line (or use the regression
button on your logger pro) and determine where the line
crosses the x axis. The point at which the line
crosses the x axis represents the molar concentration of
sucrose with a water potential that is equal to the
potato tissue water potential. At this
concentration there is no net gain or loss of water from
the potato cells.
2. By graphing the data of the percent change in mass
of the potato cores, it can be determined that the water
potential of potato cells is equal to the water
potential of an unknown sugar solution molarity.
Determine that molarity.
3. Using the explanation above the water
potential of the potato cells can be determined.
4. If the potato is allowed to dehydrate by
sitting out in the open air the water potential would
decrease (be more negative) because the concentration of
solutes within the cells would increase as potato cells
dehydrate. Therefore, the osmotic pressure and
water potential both decrease.